Blue Cone Monochromacy (BCM) is a rare genetic retinal disorder estimated to affect 1 in 100,000 people. It is an inherited retinal degeneration (IRD) caused by mutations in the OPN1LW/OPN1MW gene cluster, encoding long (L)- and middle (M)-wavelength sensitive (i.e. red-green) opsins [38,39,41] of the cone photoreceptor cells in the retina. The visual disabilities are serious and include reduced visual acuity, abnormal color vision, myopia, nystagmus and photophobia [11,20,21,34,52].
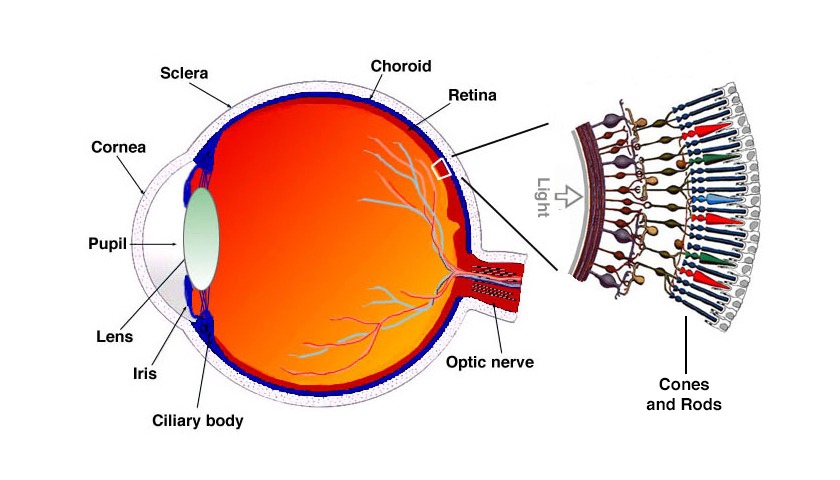
Blue Cone Monochromacy affects the retina, on the eye’s back
It is a recessive X-linked disease therefore it almost exclusively affects males (XY) while female carriers (XX) only rarely show some of the symptoms in a mild form.
Blue Cone Monochromacy is usually considered a stationary disease, with symptoms first manifesting in early infancy, although there is evidence of disease progression with macular changes associated with macular degeneration in many patients [5,6,34,52]. The first symptom observed is nystagmus in 2-3 months old newborns.
Video of a 4-months baby with Blue Cone Monochromacy showing Nystagmus.
Symptoms
There are three types of cones in the human retina that are responsible for daylight vision, visual acuity and color vision: they are sensitive to Long (red), Middle (green) and Short (blue) wavelength light[41] . When people have Blue Cone Monochromacy, both the red and green cones do not function properly, while the blue cones work normally [5,38,39]. Signs and symptoms may include low visual acuity (clarity or sharpness), impaired color vision, photophobia (light sensitivity), myopia (nearsightedness), and nystagmus (fast, uncontrollable movements of the eye).
A variety of symptoms characterize Blue Cone Monochromacy:
- Low visual acuity ranging between 20/60 and 20/200.
- Poor color discrimination (impaired ability or inability to distinguish between colours)
- Intolerance to light (characterized by difficulty seeing in bright light, especially during daylight) and associated photophobia (sensitivity to light).
- Myopia, patients often have myopia
- Nystagmus (characterized by involuntary, rhythmic eye movements) which is present since the age of 2-months and may slowly decrease with age.
For the majority of subjects with Blue Cone Monochromacy, symptoms are usually stationary. However, clinical studies show evidence of disease progression with macular changes [6,34,52].

A child with low vision, forced to read very close to the book
Synonyms:
- Blue cone monochromatism;
- S-cone monochromacy;
- Atypical X-linked achromatopsia;
- X-linked incomplete achromatopsia.
Causes: genes and mutations
There are three genes involved in Blue Cone Monochromacy, and are located at position Xq28, at the end of the q arm of the X chromosome. The three genes are in tandem and are:
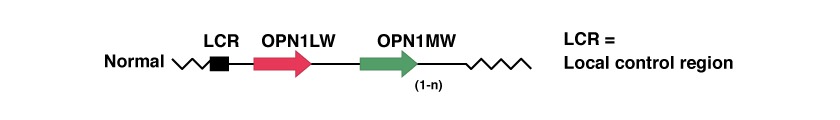
These genes encode proteins that are needed in the process of converting light into electrical signals that the brain uses for visual processing. The proteins are called L- and M-cone opsins, which play a crucial role in this process.
The Locus Control Region (LCR) serves as a promoter for the expression of two of the two genes thereafter, OPN1LW and OPN1MW, encoding opsin proteins responsible for red and green light capture in the human retina. LCR ensures exclusive expression of one opsin gene in each cone [49,54,55].
There are many genetic mutations that can affect this group of genes leading to BCM [9,17,25,26,28,38,39]: a deletion of the LCR, intragenic deletion of exons within the genes OPN1LW and OPN1MW and a 2 steps mechanism with an homologous recombination and a punctual inactivation.
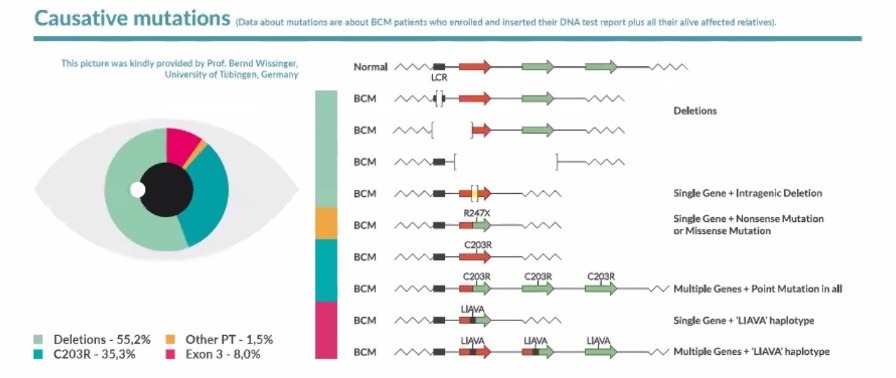
Picture taken from the he aggregated data of the BCM Patient Registry – BCMRegistry-Poster-2022-Final – [1] The BCM Patient Registry is at www.BCMRegistry.org

We can help you understand better.
Feel free to write us or join our online community.
How is it transmitted?
Blue Cone Monochromacy is transmitted genetically through genes that are passed from parents to children.
In particular, Blue Cone Monochromacy is a recessive X-linked disease which means the disease is expressed in males (XY) who are hemizygous for the mutation and in the rare females (XX) who are homozygous for the genetic mutation, i.e. in females who have the genetic mutation on both X chromosomes. It is therefore not impossible for a female (XX) to have BCM, but it is an extremely rare condition.
Diagnosis
In a male child, from 2 months upwards, an aversion to light and nystagmus may lead to the suspicion of a case of Blue Cone Monochromacy, but it does not provide sufficient indications to establish the form of the condition. To identify a case of Blue Cone Monochromacy, it is necessary to reconstruct the family history, with the condition linked to the transmission of the X chromosome, if there are other cases in the family. In adult individuals, visual acuity and color vision can be tested and a clinical diagnosis can be made. However, the most important step is the genetic confirmation through a DNA test.
The most suitable diagnostic tools are:
- A DNA test.
- A color test such as Farnsworth D-15 or Farnsworth Munsell 100 Hue test.
- The reconstruction of the family history or family Pedigree of the disease.
- The electroretinogram (ERG), which can demonstrate the loss of the functions of L/M cones with the consequent maintenance of the function of S-cones and rods[4].
It’s important consider differential diagnosis distinguishing Blue Cone Monochromacy from others diseases that present with similar clinical features, for example, achromatopsia [7]. It is important to reach the correct diagnosis because the evolution of the disease, the possible therapies and necessary aids change depending on the specific condition. The crucial step to confirm the diagnosis of Blue Cone Monochromacy is to test the DNA.
Treatments
As of today, there is no known cure for Blue Cone Monochromacy; however, the efficacy and safety of various prospective treatments are currently being evaluated.
The goal of research studies[15,16,23,51,59,61,62] is to virally supplement retinal cells expressing mutant genes associated with the Blue Cone Monochromacy phenotype with healthy forms of the gene; thus, allowing the repair and proper functioning of retinal photoreceptor cells in response to the instructions associated with the inserted healthy gene.
Additionally, corrective visual aids and personalized vision therapy provided by Low Vision Specialists may help patients correct glare and optimize their visual acuity.
Epidemiology
Blue Cone Monochromacy is a cause of inherited low vision estimated to affect approximately 1 in 100,000 people [27]. The disease affects male recipients of the X-linked mutation, while females usually remain unaffected carriers of the BCM trait.
History
Early discoveries
Blue Cone Monochromacy has been known for many years, with the first detailed description dating back to Huddart in 1777 [24], who recognized individuals affected by Blue Cone Monochromacy had difficulties in distinguishing colors, but could differentiate between white, black, and various light or bright colors.
Subsequent studies on Blue Cone Monochromacy were conducted by Sloan in 1954 [48] and Blackwell and Blackwell in 1961 [8], who described patients capable of distinguishing between blue and yellow signals that seemed to have functional rods and S-cones cells. Furthermore, Spivey in 1965 [50] indicated that affected individuals were able to see small blue objects on a large yellow field and vice versa.
The disease has also been studied also by Alpern et al. (1960) [2,3] and by Fleischman (1981) but the most important results have been obtained in 1989 and 1993 by Nathans et al. [38,39] and in 1991 by Reyniers et al. [46] who identified the genes causing Blue Cone Monochromacy.
Recent studies
Only in the last few years and thanks to the support of the BCM Families Foundation, Blue Cone Monochromacy has been studied with the aim to find a treatment.
Particularly since 2010 the BCM Families Foundation have been financing clinical studies at the University of Pennsylvania with the aim to understand if there are therapies that can be a treatment for Blue Cone Monochromacy. Positive results, never obtained before, show the presence of sufficient cone cells in the retina to warrant therapies [11]. These cone photoreceptor cells can be treated. Another study compared patients belonging to the two major Blue Cone Monochromacy causative mutations, deletions and C203R missense mutations, and identified age windows of opportunity for intervention through therapies [52]. This set of studies represents a natural history study of Blue Cone Monochromacy. Further studies have led to the identification of outcome measures for a clinical trial and the identification of inclusion and exclusion criteria [12,30,33,47,51].
With the financial support and/or the collaboration of the BCM Families Foundation, several studies on AAV vectors have been done. The group of Dr. W.W. Hauswirth at the University of Florida before and the group of Dr. Wen Tao Deng at the West Virginia University then, have been working on animal models of Blue Cone Monochromacy to identify the best AAV vector [15,16,18,23,59,61,62]. Adverum Biotechnologies. Inc, has evaluated a novel intravitreal vector for the treatment of Blue Cone Monochromacy [23]. It used ADVM-062, a vector optimized for cone-specific expression of human L-opsin. Unlike existing therapies that involve subretinal vector injection, ADVM-062 can be administered using a single intravitreal (IVT) injection, which poses less risk to the central retinal structure of BCM patients.
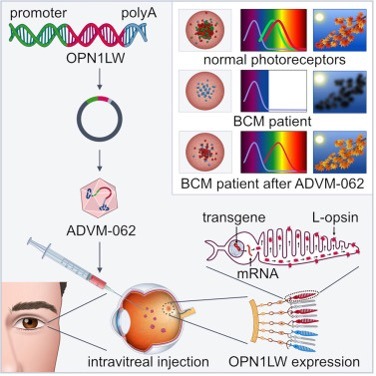
Picture taken from ref. [23]
Several recent scientific studies have been done on the Blue Cone Monochromacy. [9,17,25,26,28, 38,39,46,58] to identify all causative mutations and to deeply understand main causative mutations. Next Generation Sequencing (NGS) of DNA technologies have been introduced in labs and allow sequencing the entire exome or the entire genome. These technologies represent the future of DNA testing giving the possibility of sequencing an entire exome or genome within days at reasonable costs and enabling the detection of otherwise missed genetic disease. However, the widely utilized short-read next-generation sequencing (NGS) is inappropriate for the analysis of the OPN1LW/OPN1MW gene cluster of Blue Cone Monochromacy and a new tool has been recently developed [22].
Videos
- Video from the Low Vision Centers of Indiana, that shows symptoms of Blue Cone Monochromacy and the use of magenta filtered contact lenses:
- Video of a 4-months baby with Blue Cone Monochromacy showing Nystagmus
External resources
- The BCM Patient Registry
- OMIM page for Blue Cone Monochromacy – Online Mendelian Inheritance in Man (OMIM) is an online catalog of human genes and genetic disorders developed and maintained by the McKusick-Nathans Institute of Genetic Medicine at Johns Hopkins University.
- Orphanet – Orphanet is a European database and web portal that provides information about rare diseases to promote research and collaboration in the field.
- University of Tübingen, Germany project on Blue Cone Monochromacy
- A Colorful Cure (Forbes, 2023)
- Achromatopsia.info – Journey through the Light
- Kohl, S., Hamel, C. Clinical utility gene card for: blue cone monochromatism. Eur J Hum Genet19, 732 (2011)
- National Center for Advancing Translational Sciences
- The University of Arizona Health Sciences’ page for Blue Cone Monochromacy
- Fighting Blindness’ page for Blue Cone Monochromacy
References:
- “Patient Registry – Blue Cone Monochromacy”.
- Alpern M, Lee G B, Maaseidvaag F, Miller SS, (1971). “Colour vision in blue cone ‘monochromacy’”. Physiol. 212 (1): 211–33. doi:10.1113/jphysiol.1971.sp009318.
- Alpern M, Falls H F, Lee G B, (1960). “The enigma of typical total monochromacy”. Am. J. Ophthalmol. 50 (5): 996–1012. doi:1016/0002-9394(60)90353-6. PMID13682677.
- Andréasson, S; Tornqvist, K (1991). “Electroretinograms in patients with achromatopsia”. Acta Ophthal. (Copenh) 69 (6): 711-716. PMID 1789084. https://doi.org/10.1111/j.1755-3768.1991.tb02048.x
- Ayyagari R, Kakuk L E, Bingham E L, Szczesny J J, Kemp J, Toda Y, Felius J, Sieving P A, (2000). “Spectrum of color gene deletions and phenotype in patients with blue cone monochromacy” Genet. 107 (1): 75–82. doi:10.1007/s004390000338.
- Ayyagari R, Kakuk L E, Coats C L, Bingham EL, Toda Y, Felius J, Sieving P A, (1999).”Bilateral macular atrophy in blue cone monochromacy (BCM) with loss of the locus control region (LCR) and part of the red pigment gene”. Mol. Vis. 28: 5-13. PMID 10427103.
- Berson EL, Sandberg MA, Rosner B, Sullivan PL, (1983). “Color plates to help identify patients with blue cone monochromatism”. Am. J. Ophthalmol. 95 (6): 741–7. doi:1016/0002-9394(83)90058-2. PMID6602551.
- Blackwell H R, Blackwell O M, (1961). “Rod and cone receptor mechanisms in typical and atypical congenital achromatopsia”. Vision Res. 1 (1–2): 62–107. doi:1016/0042-6989(61)90022-0.
- Buena-Atienza E, Nasser F, Kohl S, Wissinger B, (2018) “A 73,128 bp de novo deletion encompassing the OPN1LW/OPN1MW gene cluster in sporadic Blue Cone Monochromacy: A case report.” BMC Med. Genet. 19, 107 (2018). – DOI: 1186/s12881-018-0623-8
- Carroll J, et al. (2010) “Deletion of the X-linked opsin gene array locus control region (LCR) results in disruption of the cone mosaic” Vision Res. 50, 1989–1999 (2010). – doi: 1016/j.visres.2010.07.009
- Cideciyan A V, Hufnagel R B, Carroll J, Sumaroka A, Luo X, Schwartz S B, Dubra A, Land M, Michaelides M, Gardner J C, Hardcastle A J, Moore A T, Sisk R A, Ahmed Z M, Kohl S, Wissinger B, Jacobson S G, (2013) “Human cone visual pigment deletions spare sufficient photoreceptors …”. Hum Gene Ther 2013; 24: 993-1006. – DOI: 1089/hum.2013.153
- Cideciyan A V, Roman A J, Jacobson S G, Yan B, Pascolini M, Charng J, Pajaro S, Nirenberg S, (2016) “Developing an outcome measure with high luminance for optogenetics treatment of severe retinal degenerations and …”. Invest Ophthalmol Vis Sci. 57(7):3211-21. DOI: 1167/iovs.16-19586
- Crognale M A, Fry M, Highsmith J, Haegerstrom-Portnoy G, Neitz M, Neitz J, Webster M A, (2004). “Characterization of a novel form of X-linked incomplete achromatopsia”. Neurosci. 21 (3): 197-203. DOI: 10.1017/s0952523804213384
- Deeb S S, (2005). “The molecular basis of variation in human color vision”. Genet. 67 (5): 369-377. DOI: 10.1111/j.1399-0004.2004.00343.x
- Deng WT, Li J, Zhu P, Chiodo V A, Smith W C, Freedman B, Baehr W, Pang J, Hauswirth W W. (2018) ‘Human L- and M-opsins restore M-cone function in a mouse model for human blue cone monochromacy’. Molecular Vision (2018) 24, 17-28. PMID: 29386880.
- Deng WT, Li J, Zhu P, Freedman B, Smith W C, Baehr W, Hauswirth W W, (2019) “Rescue of M-cone Function in Aged Opn1mw−/− Mice, a Model for Late-Stage Blue Cone Monochromacy”. Investigative Ophthalmology & Visual Science August (2019) Vol.60, 3644-3651. PMID: 31469404.
- Buena-Atienza E, Rüther K, Baumann B et al. (2016) “De novo intrachromosomal gene conversion from OPN1MW to OPN1LW in the male germline results in Blue Cone Monochromacy”. Sci Rep 6, 28253 (2016). DOI: 10.1038/srep28253.
- Emily R. Sechrest, Kathryn Chmelik, Wendy D. Tan, Wen-Tao Deng, (2023) “Blue cone monochromacy …” Vision Research,Volume 208,2023,108221,ISSN 0042-6989, – DOI: 1016/j.visres.2023.108221
- Gardner J C, Liew G, Quan Y H, Ermetal B, Ueyama H, Davidson A E, Schwarz N, Kanuga N, Chana R, Maher E, Webster A R, Holder G E, Robson A G, Cheetham, M E, Liebelt J, Ruddle J B, Moore A T, Michaelides M, Hardcastle A J, (2014). “A three different cone opsin gene array mutational mechanisms with genotype-phenotype correlation and functional investigation of cone opsin variants”. Mutat. 35 (11): 1354-1362. DOI: 10.1002/humu.22679
- Gardner J C, Webb T R, Kanuga N, Robson A G, Holder G E, Stockman A, Ripamonti C, Ebenezer N D, Ogun O, Devery S, Wright G A, Maher E R, Cheetham M E, Moore A T, Michaelides M, Hardcastle A J, (2010). “X-Linked Cone Dystrophy Caused by Mutation of the Red and Green Cone Opsins”. J. Hum. Genet. 87 (1): 26–39. doi:10.1016/j.ajhg.2010.05.019.
- Gardner J C, Michaelides M, Holder G E, Kanuga N, Webb T R, Mollon J D, Moore A T, Hardcastle A J, (2009). “Blue cone monochromacy: Causative mutations and associated phenotypes”. Molecular Vision. 15: 876–884. PMC2676201. PMID 19421413.
- Haer-Wigman L, den Ouden A, van Genderen MM, Kroes HY, Verheij J, Smailhodzic D, Hoekstra AS, Vijzelaar R, Blom J, Derks R, Tjon-Pon-Fong M, Yntema HG, Nelen MR, Vissers LELM, Lugtenberg D, Neveling K. (2022) “Diagnostic analysis of the highly complex OPN1LW/OPN1MW gene cluster using long-read sequencing and MLPA.” NPJ Genom Med. 2022 Nov 9;7(1):65. doi: 10.1038/s41525-022-00334-9.
- Hanna K, Nieves J, Dowd C, Bender K O, Sharma P, Singh B, Renz M, Ver Hoeve J N, Cepeda D, Gelfman C M, Riley B E, Grishanin R N, (2023) “Preclinical evaluation of ADVM-062, a novel intravitreal …vector for the treatment of blue cone monochromacy”. Mol Ther. 2023 Jul 5;31(7):2014-2027. doi: 10.1016/j.ymthe.2023.03.011.
- Huddart, J (1777). “An account of persons who could not distinguish colours”. Philos. Trans. R. Soc. 67: 260. doi:1098/rstl.1777.0015. S2CID186212155.
- Katagiri S., et al. , (2018) “Genotype determination of the OPN1LW/OPN1MW genes: Novel disease-causing mechanisms in Japanese patients with blue cone monochromacy”. Sci. Rep. 8, 11507 (2018). DOI: 1038/s41598-018-29891-9
- Keller U, Wissinger B, Tippmann S, Kohl S, Kraus H, Foerster M H, (2004). “Blue cone monochromatism: clinical findings in patients with mutations in the red/green opsin gene cluster”. Graefes Arch. Clin. Exp. Ophthalmol. 242 (9): 729-735. DOI: 1007/s00417-004-0921-z .
- Kohl S, Hamel C P, (2011). “Clinical utility gene card for: blue cone monochromatism”. Eur. J. Hum. Genet. 19 (6): 732. doi:1038/ejhg.2010.232.
- Ladekjaer-Mikkelsen, A S; Rosenberg, T; Jørgensen, A L (1996). “A new mechanism in blue cone monochromatism”. Hum. Genet. 98 (4): 403–408. doi:1007/s004390050229.
- Lewis R A, Holcomb J D, Bromley W C, Wilson M C, Roderick T H, Hejtmancik J F, (1987). “Mapping X-linked ophthalmic diseases: III. Provisional assignment of the locus for blue cone monochromacy to Xq28”. Arch. Ophthalmol. 105 (8): 1055-1059. DOI: 1001/archopht.1987.01060080057028
- Luo X, Cideciyan A V, Iannaccone A, Roman A J, Ditta L C, Jennings B J, Yatsenko S, Sheplock R, Sumaroka A, Swider M, Schwartz S B, Wissinger B, Kohl S, Jacobson S G, (2015). “Blue cone monochromacy: visual function and efficacy outcome measures for clinical trials”. PLOS ONE. 10 (4): e0125700 doi:1371/journal.pone.0125700.
- Mancuso K, Hauswirth W W, Li Q, Connor T B, Kuchenbecker J A, Mauck M C, Neitz J, Neitz M, (2009). Nature (2009) 461:784-787. PMID: 19759534.
- Mancuso K, Mauck M C, Kuchenbecker J A, Neitz M, and Neitz J, (2010) “A Multi-Stage Color Model Revisited…” 2 (2010) R.E. Anderson et al. (eds.), Retinal Degenerative Diseases, Advances in Experimental Medicine and Biology 664. PMID: 20238067.
- Mascio A A, Roman A J, Cideciyan A V, Sheplock R, Wu V, Garafalo A V, Sumaroka A, Pirkle S, Kohl S, Wissinger B, Jacobson S G and Barbur J L, (2023) “Color vision in blue cone monochromacy: outcome measures for a clinical trial” Transl Vis Sci Techno 2023 Jan 3;12(1):25 DOI: 1167/tvst.12.1.25
- Michaelides M, Johnson S, Simunovic M P, Bradshaw K, Holder G, Mollon J D, Moore A T, Hunt D M, (2005). “Blue cone monochromatism: a phenotype and genotype assessment with evidence of progressive loss of cone function in older individuals”. Eye (Lond). 19 (1): 2–10. doi:1038/sj.eye.6701391. PMID15094734.
- Mizrahi-Meissonnier L, Merin S, Banin E, Sharon D, (2010). “Variable Retinal Phenotypes caused by mutations in the X-linked phopigment gene array”. Inv. Ophthal. Vis. Sci. 51 (8): 3884-92. PMID 20220053.
- Nathans, J (1987). “Molecular biology of visual pigments”. Rev. Neurosci 10: 163-194. PMID 3551758.
- Nathans, J (1999). “The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments”. 24 (2): 299-312. PMID 10571225
- Nathans, J; Davenport, C M; Maumenee, I H; Lewis, R A; Hejtmancik, J F; Litt, M; Lovrien, E; Weleber, R; Bachynski, B; Zwas, F; Klingaman, R; Fishman, G (1989). “Molecular genetics of human blue cone monochromacy”. Science. 245 (4920): 831–838. doi:1126/science.2788922.
- Nathans J, Maumenee I H, Zrenner E, Sadowski B, Sharpe L T, Lewis R A, Hansen E, Rosenberg T, Schwartz M, Heckenlively J R, Trabulsi E, Klingaman R, Bech-Hansen N T, LaRoche G R, Pagon R A, Murphey W H, Weleber R G, (1993). “Genetic heterogeneity among blue cone monochromats”. J. Hum. Genet. 53 (5): 987–1000. PMC1682301. PMID 8213841.
- Nathans J, Piantanida T P, Eddy R L, Shows T B, Hogness D S, (1986). “Molecular genetics of inherited variation in human color vision”. Science 232 (4747): 203-210. PMID 3485310.
- Nathans J, Thomas D, Hogness D S, (1986). “Molecular genetics of human color vision: the genes encoding blue, green, and red pigments”. Science 232 (4747): 193-202. PMID 2937147
- Neitz J, Neitz M, (2011). “The genetics of normal and defective color vision”. Vision Res. 51 (7): 633–651. doi:1016/j.visres.2010.12.002.
- Neitz M, (2000). “Molecular Genetics of Color Vision and Color Vision Defects”. Archives of Ophthalmology. 118 (5): 691–700. doi:1001/archopht.118.5.691.
- Pinckers A, (1992) “Berson test for blue cone monochromatism”. Int. Ophthalmol. 16 (3):185-186. PMID 1452423.
- Reitner A, Sharpe L T, Zrenner E, (1991). “Is colour vision possible with only rods and Blue sensitive cones?”. Nature. 352 (6338): 798–800. Bibcode:352..798R. doi:10.1038/352798a0. PMID1881435. S2CID 4328439
- Reyniers E, et al. , (1995) “Gene conversion between red and defective green opsin gene in blue cone monochromacy”. Genomics 29, 323–328 (1995). – PubMed
- Semenov E P, Sheplock R, Roman A J, McGuigan D B, Swider M, Cideciyan A V, Jacobson S G, (2022) “Reading performance in blue cone monochromacy: defining an outcome measure for a clinical trial.” Transl Vis Sci Technol. 2020 Dec 8;9(13):13. – doi: 1167/tvst.9.13.13
- Sloan L L, Newhall S M, (1942). “Comparison of cases of atypical and typical achromatopsia”. American Journal of Ophthalmology. 25 (8): 945–961. doi:1016/S0002-9394(42)90594-4.
- Smallwood P M, Wang Y, Nathans J, (2002) “Role of a locus control region in the mutually exclusive expression of human red and green cone pigment genes”. Proc. Natl. Acad. Sci. U.S.A. 99, 1008–1011 (2002). – PMC – PubMed
- Spivey B E, (1965) “The X-linked recessive inheritance of atypical monochromatism”. Arch. Ophthalmol. 74 (3): 327–333. doi:1001/archopht.1965.00970040329007.
- Sumaroka A, Cideciyan AV, Sheplock R, Wu V, Kohl S, Wissinger B, Jacobson SG, (2020) “Foveal therapy in blue cone monochromacy: predictions of visual potential from artificial intelligence”. Front Neurosci. 2020 Aug 3;14:800. doi: 10.3389/fnins.2020.00800.
- Sumaroka A, Garafalo AV, Cideciyan AV, Charng J, Roman AJ, Choi W, Saxena S, Aksianiuk V, Kohl S, Wissinger B, Jacobson SG. (2018) “Blue cone monochromacy caused by the C203R missense mutation or large deletion mutations”. Invest Ophthalmol Vis Sci. 2018 Dec 3;59(15):5762-72. doi:https://doi.org/10.1167/iovs.18-25280
- Wang C., et al. , Novel OPN1LW/OPN1MW deletion mutations in 2 Japanese families with blue cone monochromacy. Hum. Genome Var. 3, 16011 (2016). – PMC – PubMed
- Wang Y., et al. , A locus control region adjacent to the human red and green visual pigment genes. Neuron 9, 429–440 (1992). – PubMed
- Wang, Y; Macke, J P; Merbs, S L; Zack, D J; Klaunberg, B; Bennett, J; Gearhart, J; Nathans, J (1992). “A locus control region adjacent to the human red and green visual pigment genes”. 9 (3): 429-440. PMID 1524826.
- Weale, RA (1953). “Cone Monochromatism”. The Journal of Physiology. 121 (3): 548–569. doi:1113/jphysiol.1953.sp004964.
- Winderickx J, Sanocki E, Lindsey DT, Teller DY, Motulsky AG, Deeb SS, (1992). “Defective colour vision associated with a missense mutation in the human green visual pigment gene”. Nat. Genet. 1 (4): 251–6. doi:1038/ng0792-251.
- Wissinger B, Baumann B, Buena-Atienza E, Ravesh Z, Cideciyan AV, Stingl K, Audo I, Meunier I, Bocquet B, Traboulsi EI, Hardcastle AJ, Gardner JC, Michaelides M, Branham KE, Rosenberg T, Andreasson S, Dollfus H, Birch D, Vincent AL, Martorell L, Català Mora J, Kellner U, Rüther K, Lorenz B, Preising MN, Manfredini E, Zarate YA, Vijzelaar R, Zrenner E, Jacobson SG, Kohl S, (2022) “The landscape of submicroscopic structural variants at the OPN1LW/OPN1MW gene cluster on Xq28 underlying blue cone monochromacy”. Proc Natl Acad Sci U S A. 2022 Jul 5;119(27):e2115538119. doi: 10.1073/pnas.2115538119.
- Xie B, Nakanishi S, Guo Q, Xia F, Yan G, An J, Li L, Serikawa T, Kuramoto T, Zhang Z. (2010) ”A novel middle-wavelength opsin (M-opsin) null-mutation in the retinal cone dysfunction rat”. Exp. Eye Res. (2010) 91 (1):26-33. PMID: 20371244.
- Yatsenko S. A., et al. , “High-resolution microarray analysis unravels complex Xq28 aberrations in patients and carriers affected by X-linked blue cone monochromacy”. Clin. Genet. 89, 82–87 (2016). – PMC – PubMed
- Zhang Z, Pang J, Xia F, Guo Q, Li L, An J, Zhang L, Hauswirth W W, Yang S, Li Z. “… A Model For Blue Cone Monochromacy”. PMID: 29386880.
- Zhang Y, Deng W T, Du W, Zhu P, Li J, Xu F, Sun J, Gerstner C D, Baehr W, Boye Sanford L, Zhao C, Hauswirth W W, Pang J, (2017) “…Therapy in a Mouse Model of Blue Cone Monochromacy”. Sci Rep 7, 6690 (2017). https://doi.org/10.1038/s41598-017-06982-7


